Categories
Change Password!
Reset Password!


For low back pain, the efficacy and safety of dual fixed-dose combination of Etoricoxib and Thiocolchicoside is comparable to triple fixed-dose combination of Chlorzoxazone, Diclofenac, and Paracetamol.
In a randomized active-controlled trial, both the dual fixed-dose combination (DFC) of Etoricoxib (60 mg) and Thiocolchicoside (4 mg) and the triple fixed-dose combination (TFC) of Chlorzoxazone (500 mg), Diclofenac (50 mg), and Paracetamol (325 mg) exhibited similar effectiveness and safety profiles for treating recent-onset low back pain (LBP). However, the DFC showed a significant advantage over TFC in patients with very severe pain and functional disability.
Investigators aimed to contrast the safety and effectiveness of DFC and TFC for LBP. Overall, 200 eligible adult participants (18 to 70 years old), battling with LBP and muscle spasms lasting up to 14 days and who had a Wong-Baker Faces Pain score of more than 4, were included in the study after procuring written informed consent. The recruited subjects were randomly allocated in a 1:1 ratio to receive treatment with either DFC or TFC for 28 days.
The assessment of effectiveness involved measuring changes in scores from baseline (prior to the intervention) to day 28 using the Wong-Baker Faces Pain Scale and the Oswestry Disability Index (ODI) questionnaire, along with determination of the proportion of participants who showed improvement with the treatment. Evaluation of safety was done on the basis of the occurrence of adverse events and the results of clinical laboratory tests.
After undergoing treatment with either DFC or TFC, there was a notable reduction in pain intensity and enhancement in functional ability. The decline in Wong-Baker Faces Pain score and ODI from the initial values was similar for both intervention groups. However, a higher percentage of individuals who initially had very severe pain experienced a 30% or greater enhancement with DFC compared to TFC (approximately 25% vs. about 12%).
Additionally, a substantially greater number of crippled individuals with severe functional disability in the DFC group demonstrated improvement in comparison with the TFC group (approximately 26% vs. only 4%) (Figure 1).
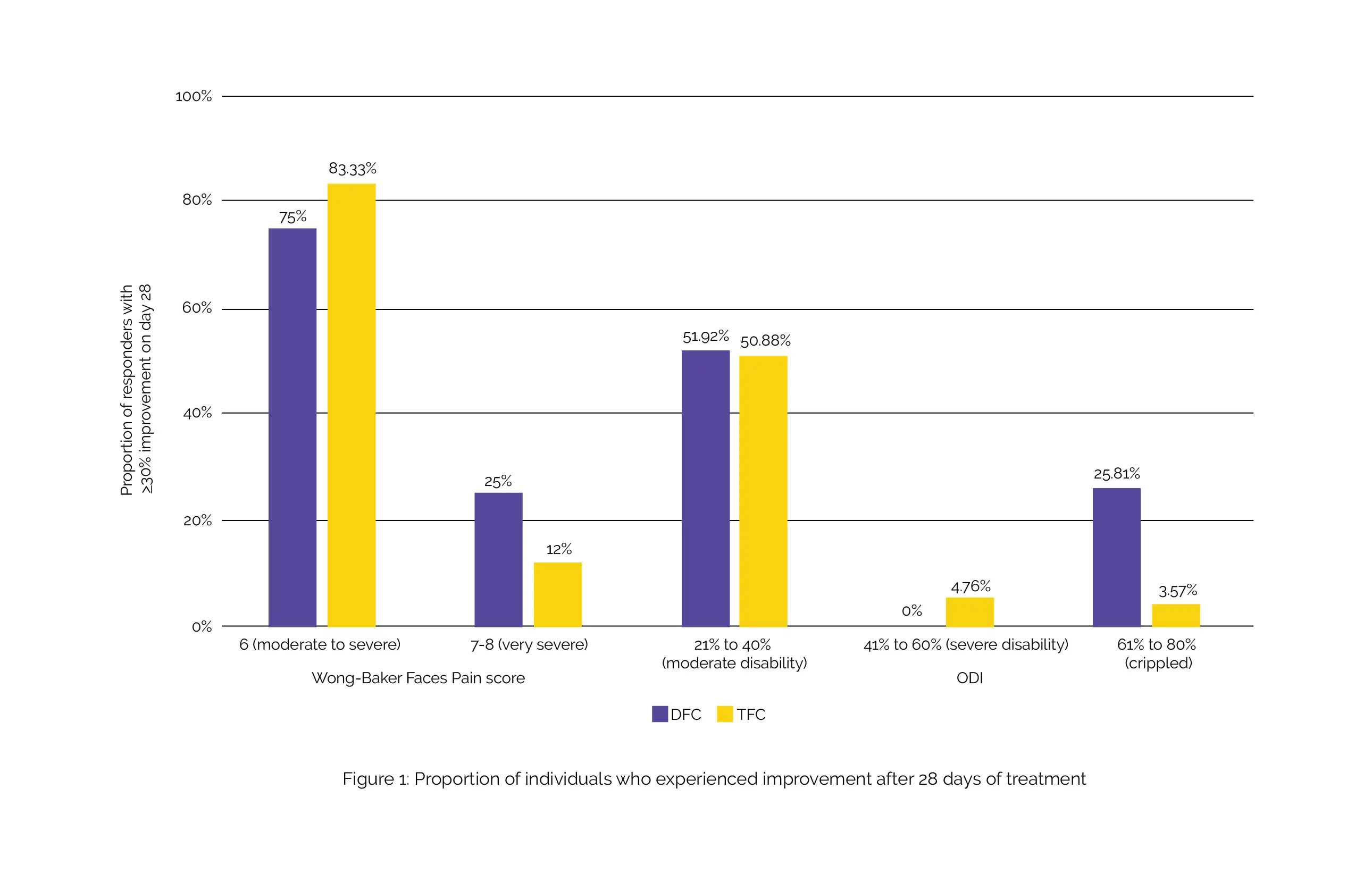
Importantly, there were no reports of adverse events or statistically significant abnormalities in laboratory test results. In the treatment of recent-onset LBP, both DFC and TFC demonstrated similar effectiveness and safety. Nevertheless, a noticeably higher number of individuals with very severe pain or functional disability exhibited enhancement following 28 days of intervention with DFC, as opposed to those treated with TFC.
Cureus
The Efficacy and Safety of a Combination of Thiocolchicoside and Etoricoxib in Low Back Pain (ESCoTEL): A Randomized Active-Controlled Trial
Arnab Karmakar et al.
Comments (0)