Categories
Change Password!
Reset Password!


For intravenous labor analgesia, Remifentanil at a relatively elevated dose in the active phase is safe and more effective when compared to the fixed lower dose regimen.
In a recent clinical trial published in the "Journal of Pain Research", researchers have delved into the realm of intravenous labor analgesia, presenting an alternative to epidural analgesia for expectant mothers with contraindications. Employing an increased Remifentanil dose during the active phase of the initial labor stage, as opposed to a fixed lower dose administered through patient-controlled analgesia, yielded improved analgesia outcomes, heightened sedation effectiveness, and elevated satisfaction scores.
The investigation focused on comparing the safety and efficacy of two distinct intravenous Remifentanil dosage strategies during the initial stage of labor. A pool of 115 parturients, ineligible for epidural analgesia but open to systemic labor pain relief, were enlisted for the study. These participants were randomly divided into two groups: Group A, which received a consistent Remifentanil dose throughout the initial labor stage, and Group B, which underwent an escalating Remifentanil dosage specifically during the active phase of labor.
Both groups received the dosages through patient-controlled analgesia. The study scrutinized various aspects including maternal pain scores using the numerical rating scale (NRS), occurrences of oxygen desaturation, sedation efficiency, participant satisfaction, as well as potential adverse reactions experienced by both mother and fetus. Notably, the study findings indicated that the mean NRS pain scores prior to analgesia and during the latent phase displayed no notable variance between the two groups.
However, during the active phase of labor, Group B showcased remarkably reduced mean NRS pain scores, achieving the lowest pain score in comparison to Group A. Additionally, Group B displayed elevated scores for overall sedation and satisfaction in contrast to Group A. No noteworthy distinction was observed between the two groups in terms of the duration of the initial labor stage, the quantity of boluses administered, and the overall consumption of Remifentanil (Table 1).
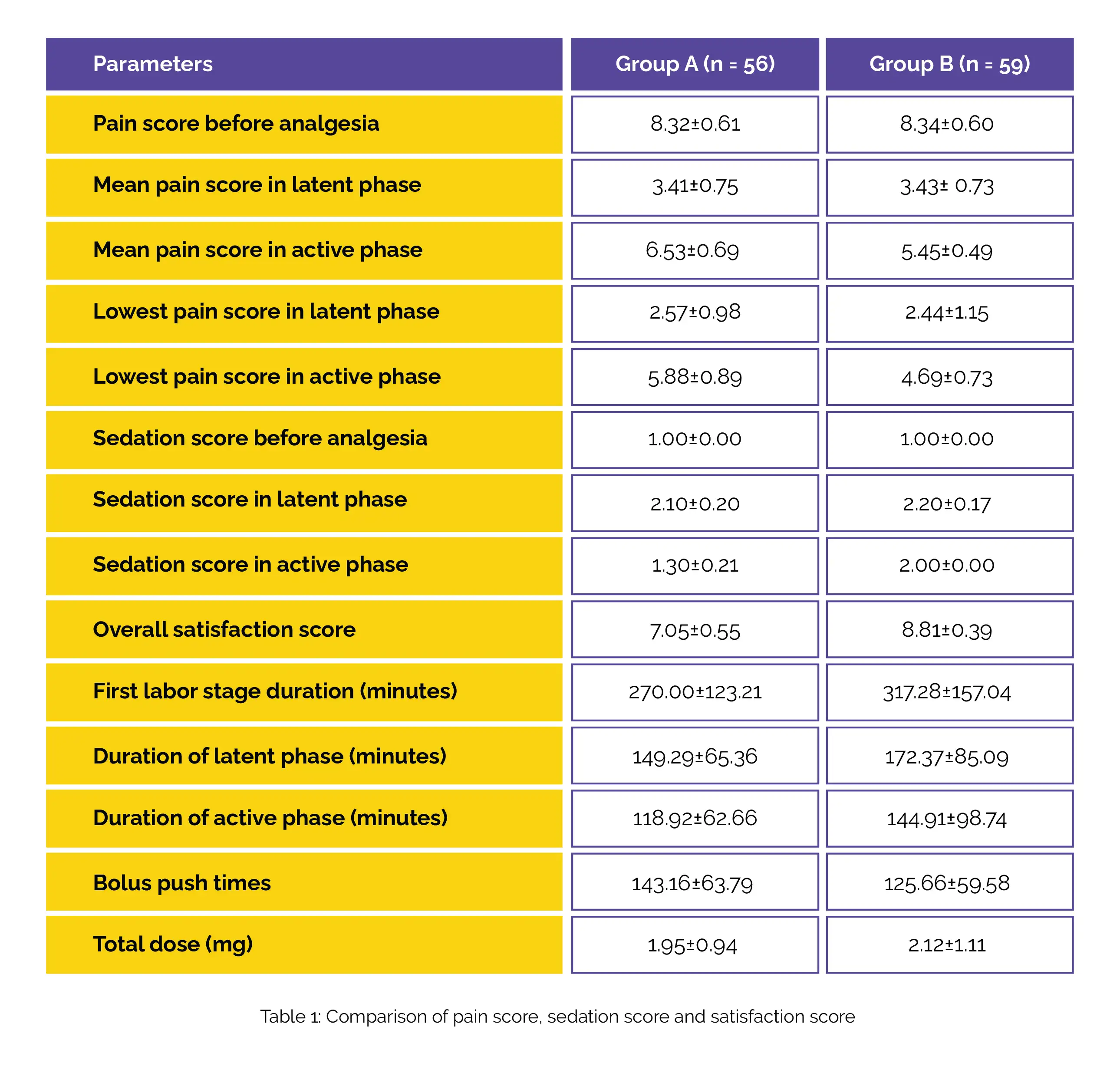
Importantly, the incidence of adverse reactions remained similar between the two groups. As a conclusion drawn from the research, it was highlighted that employing a relatively heightened intravenous Remifentanil dosage, coupled with patient-controlled analgesia administration during the active labor phase, proved to be not only safe but also more effective than adhering to a fixed-dosage regimen for labor pain management in the initial stage. This insightful study sheds light on potential advancements in pain relief strategies during childbirth for those who cannot undergo epidural analgesia.
Journal of Pain Research
Remifentanil at a Relatively Elevated Dose in Active Phase is Safe and More Suitable Than Fixed Lower Dose for Intravenous Labor Analgesia
Meng Cai et al.
Comments (0)