Categories
Change Password!
Reset Password!


Novel respiratory syncytial virus vaccine proves effective and safe in safeguarding older adults against respiratory illness.
Bivalent RSV prefusion F protein-based (RSVpreF) vaccine safely prevents respiratory syncytial virus (RSV)-linked lower respiratory tract illness and acute respiratory illness in geriatric patients aged 60 years or more, as elucidated in a study issued in ‘The New England Journal of Medicine’.
RSV is a viral infection that causes significant illness in elders. Edward E Walsh et al. explored the safety and effectiveness of a novel vaccine for this infection. The study authors investigated people aged ≥60 years in this ongoing, phase 3 trial. They were administered with one RSVpreF injection intramuscularly dosed at 120 μg (60 μg in subgroup A and 60 μg in subgroup B) or placebo.
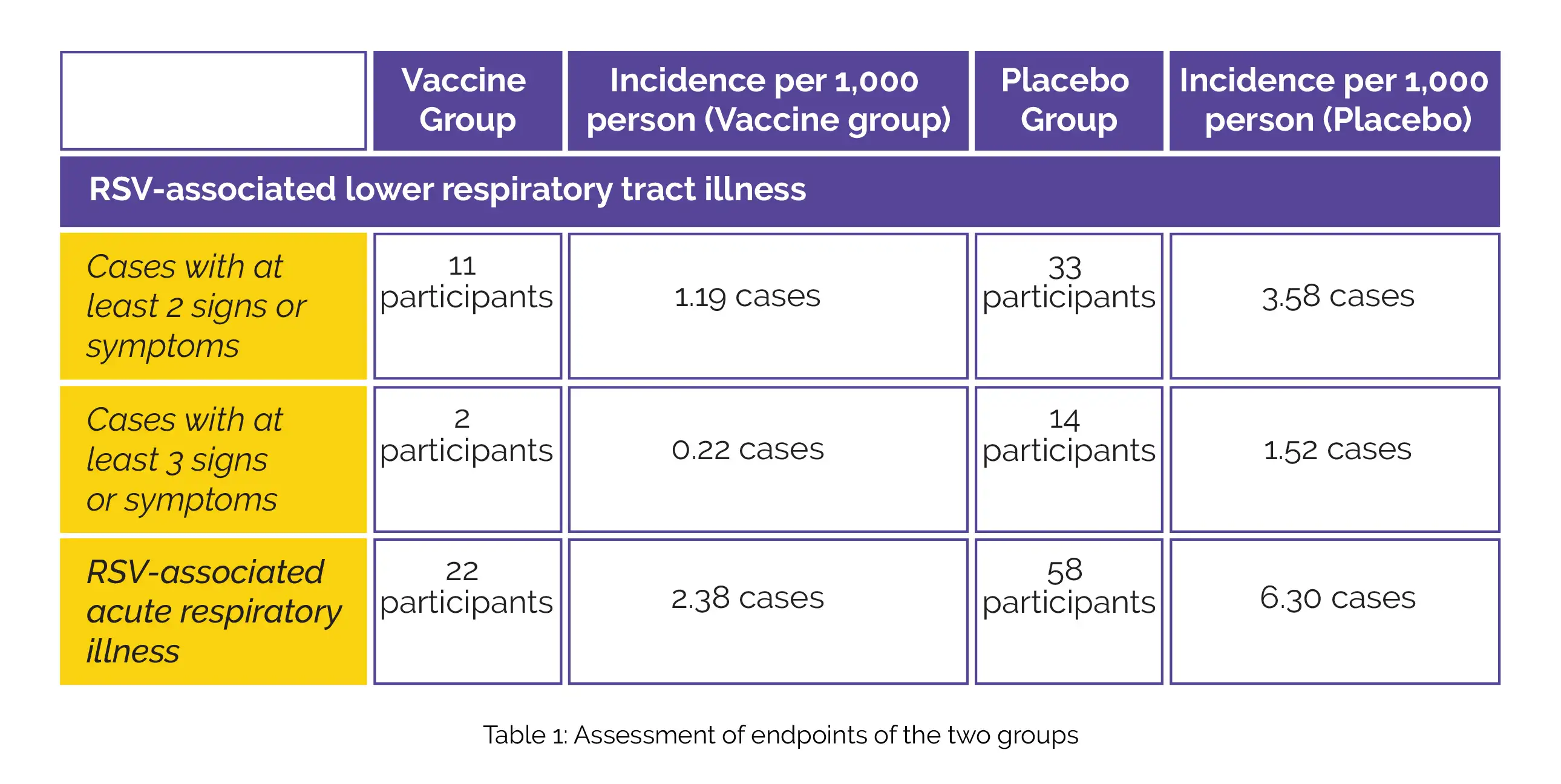
The study focused on two main endpoints: assessing the vaccine's effectiveness in preventing seasonal RSV-related lower respiratory tract illness with at least 2 or 3 symptoms. Additionally, the secondary endpoint was to evaluate the effectiveness of this investigational vaccine against RSV-linked acute respiratory illness. A total of 34,284 people were considered for this study. Out of which, 17,215 participants received the RSVpreF vaccine and 17,069 participants were in the placebo group during the interim analysis.
Details of the number of cases with RSV-linked lower respiratory tract illness with a minimum of 2 signs or symptoms (vaccine efficacy, 66.7%; 96.66% confidence interval [CI], 28.8 to 85.8) and a minimum of 3 signs or symptoms (vaccine efficacy, 85.7%; 96.66% CI, 32.0 to 98.7) has been shown in Table 1, along with the details of RSV-associated acute respiratory illness (vaccine efficacy, 62.1%; 95% CI, 37.1 to 77.9):
The incidence of local reactions was found to be higher among vaccine recipients (12%) compared to those who received the placebo (7%). However, the incidences of systemic events were similar between the two groups (27% and 26% respectively). Throughout the one-month period following injection, similar rates of adverse events were reported (vaccine: 9.0%; placebo: 8.5%), with 1.4% and 1.0% respectively considered injection-related by the investigators.
Severe or life-threatening adverse events occurred in 0.4% of placebo recipients and 0.5% of vaccine recipients. Additionally, severe adverse events were witnessed in 2.3% of participants in both the vaccine and placebo groups up until the data-cutoff date.
The New England Journal of Medicine
Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults
Edward E Walsh et al.
Comments (0)