Categories
Change Password!
Reset Password!


Oral administration of zoledronic acid bisphosphonate improved low back pain symptoms and quality of life in patients with chronic low back pain.
According to the findings of a parallel, double-blinded, pilot randomized controlled trial, oral 50 mg zoledronic acid once-a-week for six weeks effectively and safely decreased modic changes volume, and improved low back pain symptoms and quality-of-life measures in chronic low back pain people suffering from modic changes. Graham K. H. Shea et al. undertook this study to explore the efficacy and safety of zoledronic acid bisphosphate vs. placebo in 25 people having chronic low back pain and modic changes on magnetic resonance imaging.
Participants were randomized to get placebo (n = 12) or zoledronic acid (n = 13). The assessment was done at baseline, two-weeks, four-weeks, three -months and six-months for evaluating low back pain /leg pain intensity, disability (Oswestry-Disability-Index), health-related quality-of-life (RAND-36), and mental component summary scores (MCS). The type II modic alterations at baseline (56%) were prevalent.
In the zoledronic acid group, the low back pain intensity was reduced at four-weeks when compared to placebo (minimal clinically important difference [MCID] = 1.5), as shown in Table 1:
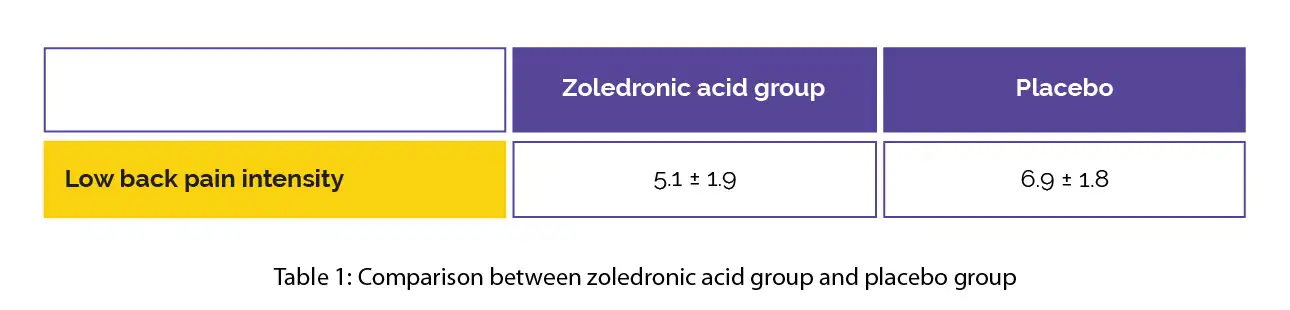
The low back pain intensity decreased at four-weeks and three-months in the zoledronic acid-treated group when compared to the baseline. Though no difference was noted in Oswestry-Disability-Index, the subscale RAND-36 metrics for physical function, energy/fatigue and pain were enhanced at three-months in comparison with the placebo, with a moderate difference for pain at six months.
The correlated mental component summary scores to baseline also enhanced at three-months and six-months by 6.9 and 6.8 respectively (MCID = 3.8). A drop in modic changes endplate affected area at the six-month follow-up was witnessed in the zoledronic acid group (-0.67 ± 0.69 cm2), while in the placebo group no alteration in size was noted (0.0 ± 0.15).
A total of 3 volunteers withdrew from the trial and no long-lasting side effects were noted. Thus, zoledronic acid bisphosphonate therapy is effective and safe for the management of chronic low back pain.
The Journal of Orthopaedic Research
Oral Zoledronic acid bisphosphonate for the treatment of chronic low back pain with associated Modic changes: A pilot randomized controlled trial
Graham K. H. Shea et al.
Comments (0)