Categories
Change Password!
Reset Password!


An interim report of phase I/II randomized controlled trial was performed to explore safety and immunogenicity of NVX-CoV2373 vaccine in healthy adult patients.
In healthy adults, 2 doses of NVX-CoV2373 administered with a 21-day interval induced robust anti-SARS-CoV-2 immune responses and exhibited acceptable safety and tolerability profile.
An interim report of phase I/II randomized controlled trial was performed to explore safety and immunogenicity of NVX-CoV2373 vaccine in healthy adult patients.
Healthy adults ≥ 20 years of age (n = 200) without a history/risk of COVID-19 infection and no previous exposure to any intervention were recruited. The participants were stratified by age (< 65 or ≥ 65 years) and were randomly given two doses of NVX-CoV2373 vaccine (n = 150) or placebo (n = 50) 21 days apart. The immunogenicity and safety evaluated by serum immunoglobulin G (IgG) levels against SARS-CoV-2 rS protein on day 36 were the major endpoints ascertained. At 4 weeks following the second dose, the primary data analysis was reported, ahead of 12-month follow-up completion.
The occurrence of adverse events (AEs) in the NVX-CoV2373 and placebo arms during 7 days following each injection is depicted in Table 1:
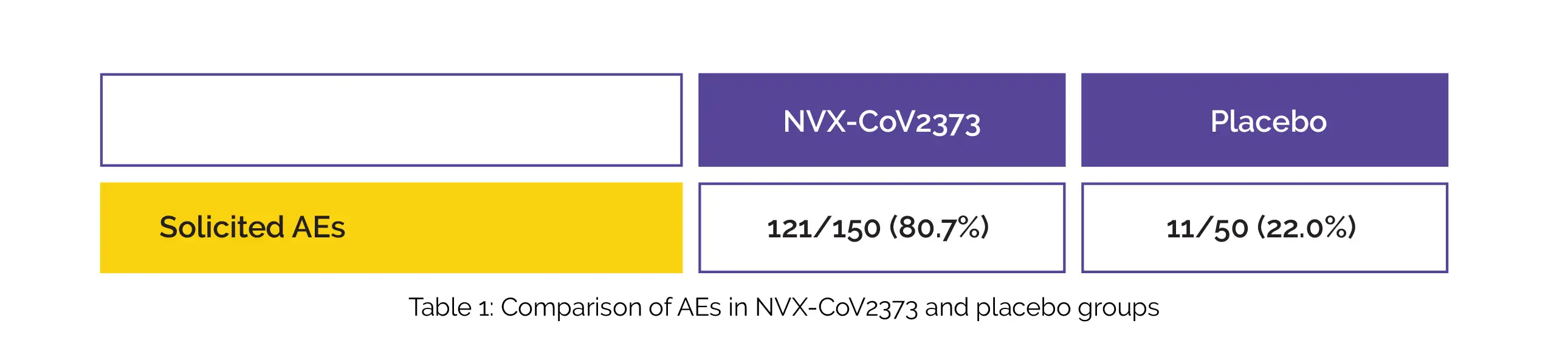
Following each vaccination, injection site pain and tenderness were the most commonly reported AEs (regardless of age) in the NVX-CoV2373 arm. By day 36, robust immune responses were witnessed in the NVX-CoV2373 arm (n = 150) such as a seroconversion rate of 100% (97.6, 100) and IgG geometric mean fold rise of 259 (219, 306). No similar response was observed in the placebo (n = 49).
NVX-CoV2373 (recombinant SARS-CoV-2 nanoparticle vaccine) was safe, well-tolerated, and elicited promising anti-SARS-CoV-2 immune responses in healthy adults.
Vaccine
Safety and immunogenicity of NVX-CoV2373 (TAK-019) vaccine in healthy Japanese adults: Interim report of a phase I/II randomized controlled trial
Taisei Masuda et al.
Comments (0)